DNA in human body is under constant attack by free radicals and various environmental factors such as UV from sunlight. Unrepaired DNA damage contributes to mutations and genome instability, and may eventually cause cancers.
To suppress tumorigenesis, cells have evolved elegant strategies to protect genome integrity, which are collectively known as DNA Damage Responses (DDRs). These strategies underlie faithful transmission of genetic materials by coupling DNA repair processes with cell proliferation and division. How DNA repair processes are exactly orchestrated has been a topic of intense research.
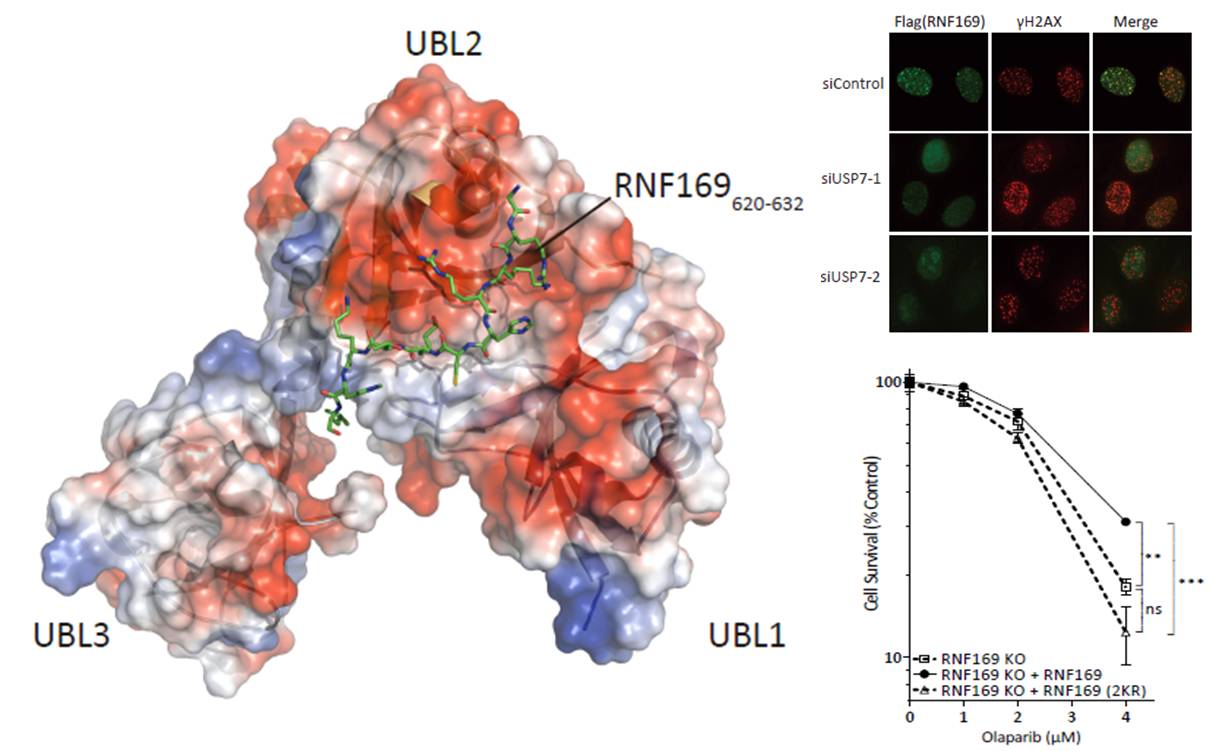
Dual-utility NLS drives RNF169-dependent DNA damage responses, image provided by Dr. Qingguo Gong
Through extensive collaboration with Dr. Michael Huen’s laboratory at The University of Hong Kong, Associate Professor GONG Qingguo and Ph.D student Mr. JIANG Yiyang from Professor SHI Yunyu’s laboratory at USTC recently uncovered an ubiquitin-dependent pathway that promotes high-fidelity DNA repair. The study entitled “Dual-utility NLS drives RNF169-dependent DNA damage responses” has been published in PNAS.
Using both structural and cellular biology methods, researchers investigated how Ubiquitin-Specific Protease 7 (USP7), an important deubiquitylating enzyme known for its role as a regulator of tumour suppressor p53, interacts with the Ring Finger protein RNF169, a DNA damage responsive factor previously identified by Huen laboratory as a key DDR regulator.
They demonstrated that the USP7-RNF169 interaction is crucial for high-fidelity DNA repair and the RNF169 and USP7 expression in breast cancer specimens showed strong links between the two proteins.
Given that USP7 inhibitors have shown promising effects in treating cancers in pre-clinical studies, and are moving into clinical trials for the treatment of certain human cancers, the study highlights the USP7-RNF169 pathway as a druggable target, and provide a strong basis to screen for small molecules that target the USP7-RNF169 interaction.
The work is supported in part by fund from The National Science Foundation of China (NSFC).
The data of crystal structure is collected at BL18U1 beamline of the National Center for Protein Science Shanghai at the Shanghai Synchrotron Radiation Facility.
Contact:
GONG Qinguo
qgg@ustcnet.
http://bionmr.ustcnet./people-en.html
(School of Life Sciences)