Dr. CHEN Wei and CUI Ping, from International Center for Quantum Design of Functional Materials (ICQD) at Hefei National Laboratory for Physical Sciences at the Microscale, enhance the hydrogen activation reactivity of nonprecious metal via confined catalysis underneath graphene and thus predict that graphene-covered Ni would be perfect platform to produce hydrogen, with cooperation with peers from the University of Science and Technology of China (USTC), Harvard University, Wuhan University.
Hydrogen, as ideal clean energy, has received a lot of attention, with its prospect of mitigating energy crisis and environment pollution. However, in the hydrogen evolution reaction (HER), the precious metal Pt is the most efficient catalyst, since the reactivity as a function of the hydrogen adsorption energy on different metal substrates follows a well-known volcano curve, peaked at Pt. Therefore, turning nonprecious metals into efficient catalysts is a fundamental challenge.
This study provides a new perspective for economical production of hydrogen and other important chemical reactions of industrial significance, by integrating the concepts of marginal catalysis and confined catalysis. The findings demonstrate that graphene-covered Ni is an appealing effective, stable, and economical catalytic platform for HER.
Confined catalysis, proposed by research team led by Dr. BAO Xinhe at Dalian Institute Chemical Physics, Chinese Academy of Sciences, demonstrates significant advantage. In confined catalysis, the whole or parts of the catalyst, reactants and products are confined in a restricted space and then the reactivity is enhanced due to modification of the relevant electronic states by the boundary conditions.
USTC research team further put up marginal catalysis, where the reactivity is improved significantly. In marginal catalysis, researchers put a 2D overlayer on a nonprecious metal with moderate reactivity to introduce confined catalysis. Using density functional theory calculations, the team shows that when the hydrogen atom is at the interface between a graphene layer and different metals, its absorption free energy reduces to some extent, compared with that when without graphene. The highlight is that when the surface of Ni (111) is covered with graphene, hydrogen evolution reactivity has been adjusted to reach around the peak of the volcano curve. Thus, graphene-covered Ni becomes catalyst with high catalytic activity. At the same time, the diffusion rate of hydrogen remains high at the interface, ensuring the quick collection of products.
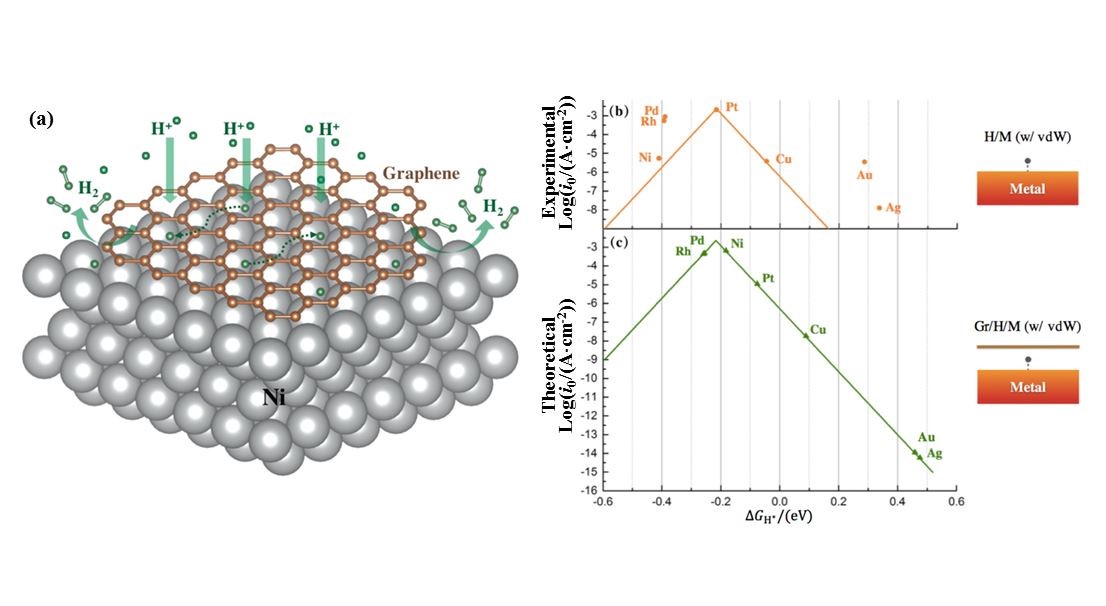
Figure. (a) Diagram of hydrogen evolution reaction catalyzed by graphene-covered Ni surface; (b) Volcano curve of catalysis activity of different metals’ surface in HER with vdW corrections; (c) Anticipated volcano curve of catalysis activity of different metals’ surface, underneath a graphene layer.(Image by ZHANG Zhenyu & CUI Ping)
The work has been published on Nano Letters, entitled “Enhancing the Hydrogen Activation Reactivity of Nonprecious Metal Substrates via Confined Catalysis Underneath Graphene” The co-first authors are ZHOU Yinong, a new bachelor degree holder from School of Physical Sciences at USTC, and Doctor CHEN Wei, a Mo-tse honored post-doctor from International Center for Quantum Design of Functional Materials (ICQD) at Hefei National Laboratory for Physical Sciences at the Microscale.
The research was sponsored by the National Natural Science Foundation of China, Chinese Academy of Sciences, Ministry of Science and Technology, Ministry of Education.
The link of the paper: http://pubs.acs.org/doi/abs/10.1021/acs.nanolett.6b02052
Corresponding author information:
Pro. ZHANG Zhenyu zhangzy@ustcnet.. http://dsxt.ustcnet./zj_ywjs.asp?zzid=1564
Dr. CUI Ping E-mail: cuipg@ustcnet..
(International Center for Quantum Design of Functional Materials (ICQD), Hefei National Laboratory for Physical Sciences at the Microscale, Synergetic Innovation Center of Quantum Information and Quantum Physics, Research Department, USTC News Center)