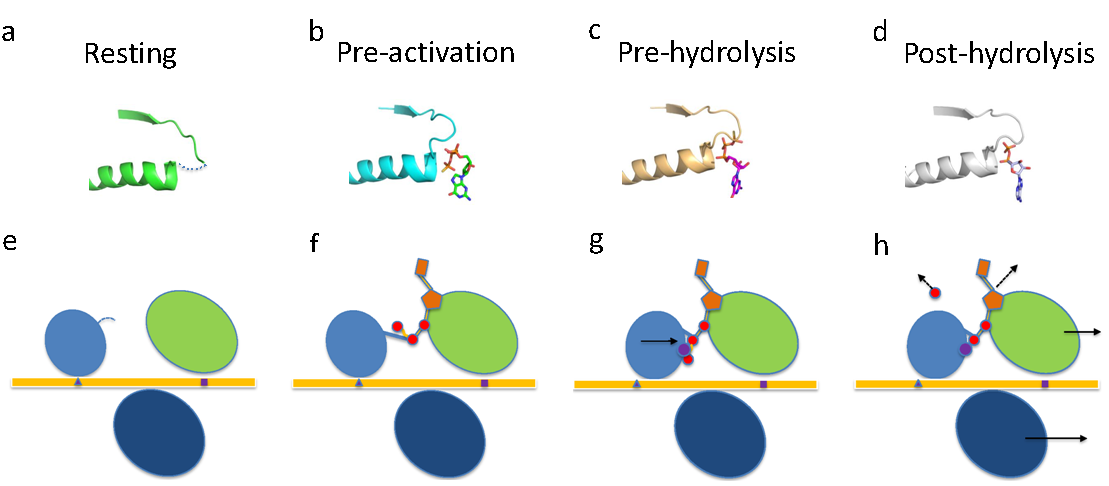
Zika virus (ZIKV) is an important emerging human pathogen. It is associated with various severe neural disorders, including a brain defect in newborn called microcephaly and an autoimmune disease known as Guillain–Barré syndrome (GBS). Therefore, investigation of the molecular mechanisms underlying ZIKV replication, assembly and host-ZIKV interactions is urgently needed to facilitate the development of anti-viral therapeutics and vaccines.By using X-ray crystallography, a group lead by Dr. JIN Tengchuan of the School of Life Sciences determined the complex structures of ZIKV NS3 helicase with GTPγS, with GTPγS–metal, and ADP-metal,representing the state of pre-activation, activated (pre-hydrolysis) and post-hydrolysis respectively. This work is recently published as a research article titled “Molecular Mechanism of Divalent-Metal-Induced Activation of NS3 Helicase and Insights into Zika Virus Inhibitor Design” on Nucleic Acids Research.
This work showed for the first timethat divalent metals are allosteric modulators of Zika virus (ZIKV) NS3 helicase.These findings provide a structural explanation for the divalent-metal-dependent conformational changes and nucleotide hydrolysis in the flavivirus NS3 helicase and probably other helicases.Furthermore, the compact conformation of NTPguides the design of novel NS3 inhibitors for ZIKV infection.
The co-first authors are 2016 graduate studentXiaocong Cao and postdoc Dr. Yajuan Li.This work is supported by the Task Force of Zika Virus Research from the Chinese Academy of Sciences (CAS). T.J. is supported by the Fundamental Research Funds for the Central Universities and the 100 Talents Program of CAS. Y.L. is supported by the China Postdoctoral Science Foundation (Grant No.: 2015M582007).
This work received wide attention by the media. Xinhua net and Science net both reported this discovery. In addition, SCIENTIA SINICA Vitae published a highlightwritten by Prof. Yan Xiang of Department of Microbiology, Immunology and Molecular Genetics, The University of Texas Health Science Center at San Antonio.
Further reading:
http://www.gov.cn/xinwen/2016-10/14/content_5119239.htm
http://news.sciencenet.cn/htmlnews/2016/10/359034.shtm
Full text article link:http://nar.oxfordjournals.org/content/early/2016/10/19/nar.gkw941.full?keytype=ref&ijkey=RHPDcyM9hwAt4Is。
ABSTRACT
Zika virus has attracted increasing attention because of its potential for causing human neural disorders, including microcephaly in infants and Guillain–Barré syndrome. Its NS3 helicase domain plays critical roles in NTP-dependent RNA unwinding and translocation during viral replication. Our structural analysis revealed a pre-activation state of NS3 helicase in complex with GTPγS, in which the triphosphate adopts a compact conformation in the absence of any divalent metal ions. In contrast, in the presence of a divalent cation, GTPγS adopts an extended conformation, and the Walker A motif undergoes substantial conformational changes. Both features contribute to more extensive interactions between the GTPγS and the enzyme. Thus, this study provides structural evidence on the allosteric modulation of MgNTP2- on the NS3 helicase activity. Furthermore, the compact conformation of inhibitory NTP identified in this study provides precise information for the rational drug design of small molecule inhibitors for the treatment of ZIKV infection.
(School of life Sciences )