The ataxia-telangiectasia mutated (ATM) protein is an apical kinase that orchestrates the multifaceted DNA-damage response as well as the most central kinase that regulates the stability of genome. Consequently, understanding of its structure provides significant insights for illustrating genome stability and developing radiosensitizers for cancer radiotherapy. However due to its large size (300-500 kDa) and structural complexity, obtaining the high-resolution structure of PIKKs (Phosphatidylinositol-3Kinase-related Kinases) has remained a challenge.
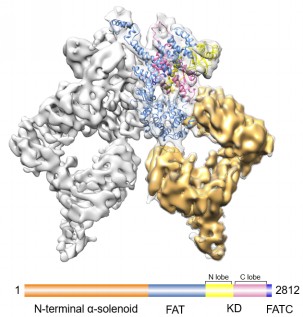
Figure:The 3D model of the intact ATM/Tel1 kinase.(Credit:WANG Xuejuan)
Now, Prof. CAI Gang’s and Prof. LIU Haiyan’s groups from University of Science & Technology of China, joining hands with Prof. WANG Weiwu’s group from Nanjing Agricultural University, revealed for the first time the fine structure of the first cryo-EM structure of ATM kinase.
Researchers acquired endogenous ATM/Tel1 directly from the yeast cells through ammonium sulfate precipitation and one-step affinity chromatography purification using IgG column. An ion exchange Mono S column was employed for further purification. Firstly negative-staining EM analysis was performed to obtain the initial model for cryo-EM refinement using Random Conical Tilt (RCT) method. Then after image processing of 57,435 carefully sorted and cleaned particles, the cryo-EM structure of the homodimeric ATM/Tel1 kinase was determined with a homology model of the C-terminal 1,027 residues of ATM/Tel1 built with Modeller using the crystal structure of mTOR catalytic core (PBD ID: 4JSV) as a template.
The 8.7-Å structure illuminated the intricate interface of ATM/Tel1 kinase homodimer and the unusual winding tertiary structure of the consecutively stacked N-terminal helical solenoid, as well as the distinct conformation of the N-terminal helical solenoid tightly packing against the FAT and kinase domains. These novel structural insights suggest that the dimer interface and the N-terminal helical solenoid could potentially allow tight regulation of the kinase activity by redundantly regulating the bindings of substrates and regulators.
The study provides a structural framework for understanding the mechanisms of ATM/Tel1 regulation as well as the development of new therapeutic agents.
This work is supported by the Ministry of Science and Technology, the Ph.D. Programs Foundation of Ministry of Education of China and Outstanding Youth Foundation of National Natural Science Foundation.
Paper Link:
http://www.nature.com/ncomms/2016/160527/ncomms11655/full/ncomms11655.html
(GUO Jianjian, USTC News Center)
GANG CAI, Ph.D. Professor
Phone/Fax: +86-0551-3603802
Email: gcai@ustcnet.