Recently, the research group of Prof. Zeng from Hefei National Laboratory for Physical Sciences at the Microscale&School of Chemistry and Materials Science has made great progress in the controlled synthesis of non-noble alloy nanocrystals. CuNi octahedra and nanocubes were successfully prepared by using borane morpholine as a reducing agent, and then were applied in the aldehyde-alkyne-amine (A3) coupling to explore the facet- and composition- dependent catalytic performance. The Cu50Ni50 nanocubes achieved the highest activity towards A3 coupling reactions. Based on theoretical calculations, the activity of catalysts is closely associated with the competition of surface energy and active sites. This work has been published on Journal of the American Chemical Society (J. Am. Chem. Soc.2015, 137 (44), pp 14027–14030) with the title of “Ratio-Controlled Synthesis of CuNi Octahedra and Nanocubes with Enhanced Catalytic Activity”. Master Menglin Wang and Doctor Liangbing Wang contributed equally.
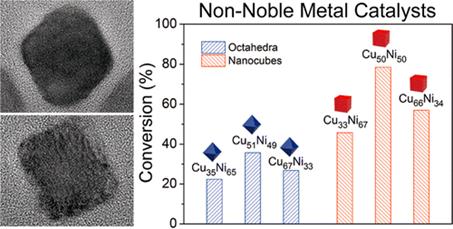
Catalytic properties towards A3 coupling over CuNi octahedra and nanocubes with tunable ratios
Non-noble metal catalysts have received considerable interest due to their low cost and abundant contents in the Earth’s crust. However, a more strenuous reaction condition is required to form non-noble alloys compared with noble ones, because the reduction potentials of non-noble metals are generally much lower than those of noble metals, meanwhile the differences in reduction potentials between different metals in the alloy are required to be eliminated. Furthermore, relatively high susceptibility of non-noble metal nanocrystals upon exposure to solution and air makes great difficulty to obtain ideal shapes with specific enclosed facets. Therefore, it remains a grand challenge in controlled synthesis of non-noble metal catalysts.
The researchers synthesized CuNi alloy octahedra and nanocubes, respectively, through manipulating the reduction kinetics in the present synthetic system based on the fact that the decomposition of borane morpholine can generates a burst of H2 molecules. In the A3 coupling, CuNi nanocubes exposed with (100) facets exhibited higher catalytic activity than octahedra exposed with (111) facets. Besides, the activity in this reaction is a volcano-type model as a function of Cu/Ni ratios. Based on the density functional theory calculations, the surface energy of (100) facets is always higher than that of (111) facets at the same molar ratio. Considering that there is a positive correlation between surface energy and catalytic activity, CuNi nanocubes are supposed to exhibit a higher activity than CuNi octahedra with the same molar ratio. At the same facets, the surface energy increases with the declined Cu/Ni ratios. However, the lowered percentage of Cu, which could provide accessible catalytically active sites for A3 will decrease the catalytic activity. Hence, the volcano-type relationship between activity and Cu/Ni ratios derives from the the competition of surface energy and active sites. This approach paves the way for rational design and synthesis of non-noble metal catalysts with controllable shapes and tunable compositions.
This work was supported by MOST of China, the National Natural Science Foundation of China, Strategic Priority Research Program B of the CAS and Fundamental Research Funds for the Central Universities.
Source: http://pubs.acs.org/doi/abs/10.1021/jacs.5b08289.